A DNAzyme‐Based Colorimetric Paper Sensor for Helicobacter pylori - Ali - 2019 - Angewandte Chemie - Wiley Online Library

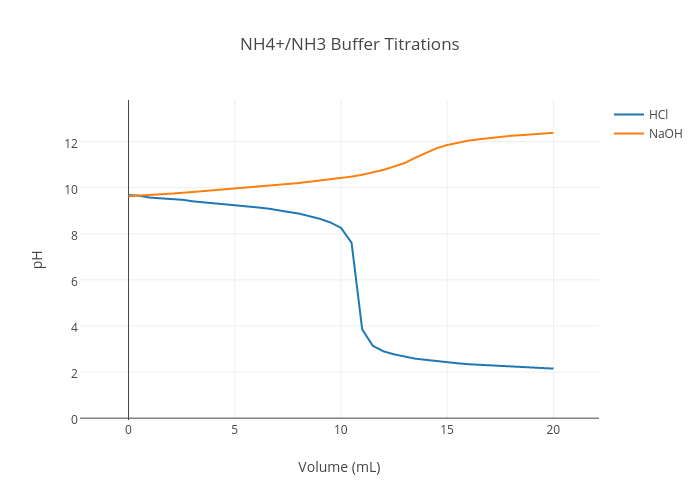
Abb. 2.4: Pufferbereiche der Pufferkapazitäten des VFA-, HCO 3 --und NH... | Download Scientific Diagram
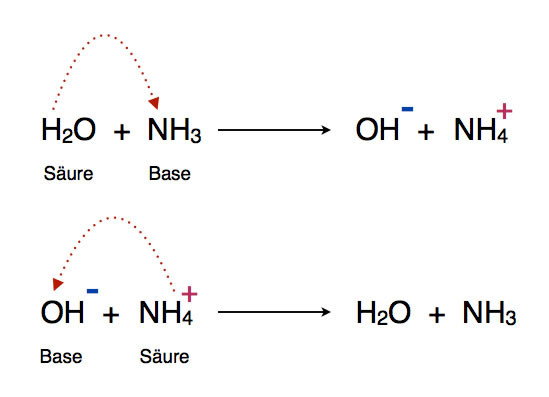
Welche Bedeutung hat die chemische Formel,,H2O+NH3- - - > OH- +NH4“ für die Säure-Base-Eigenschaften von Ammoniak? (Chemie, Säure-Base-Reaktion)
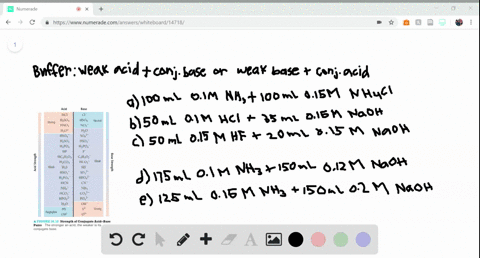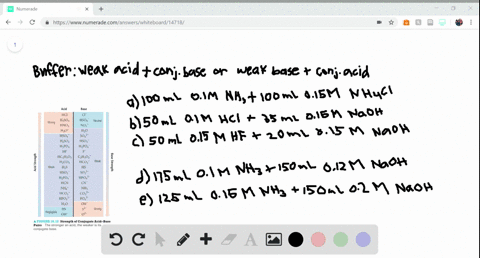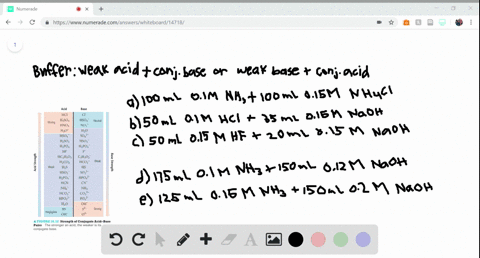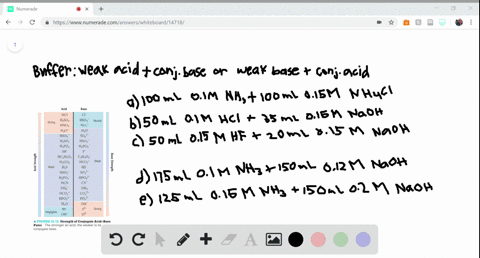
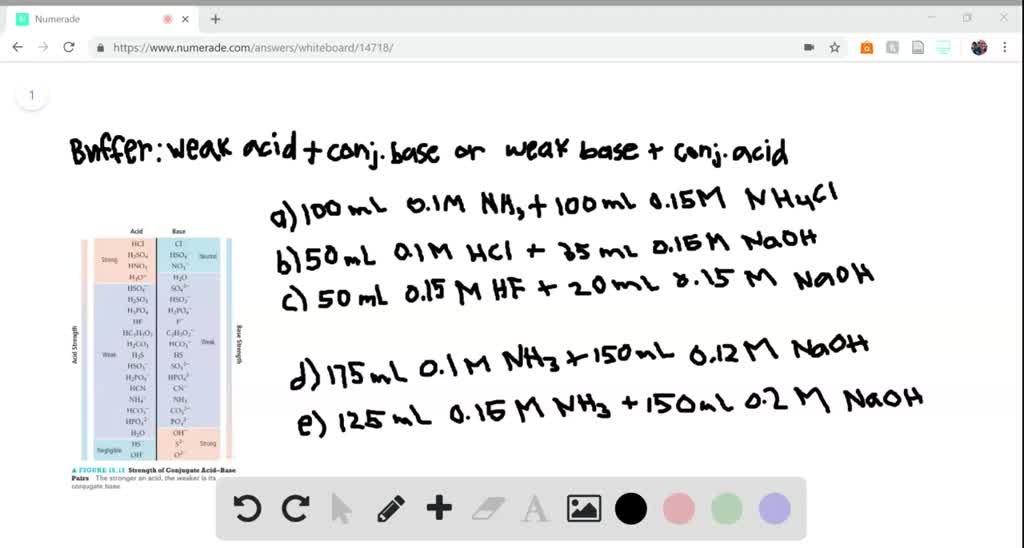
SOLVED:Determine whether the mixing of each pair of solutions results in a buffer. a. 100.0 mL of 0.10 M NH3; 100.0 mL of 0.15 M NH4Cl b. 50.0 mL of 0.10 M
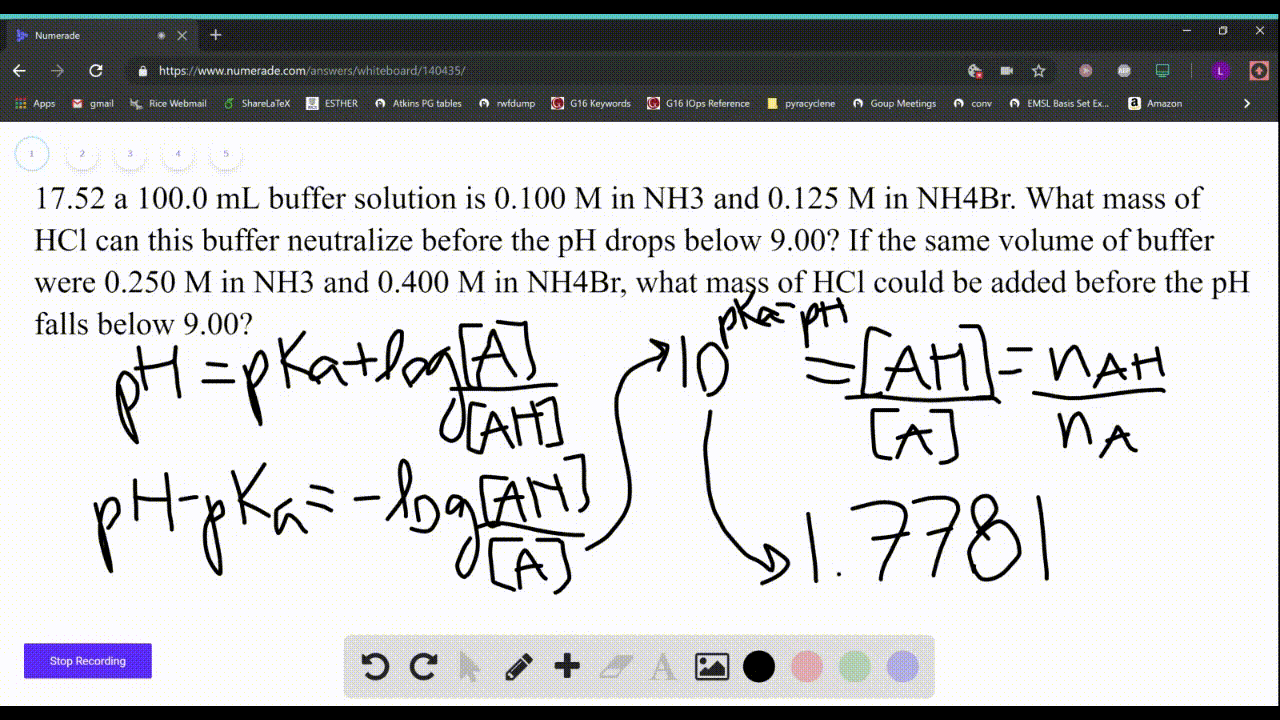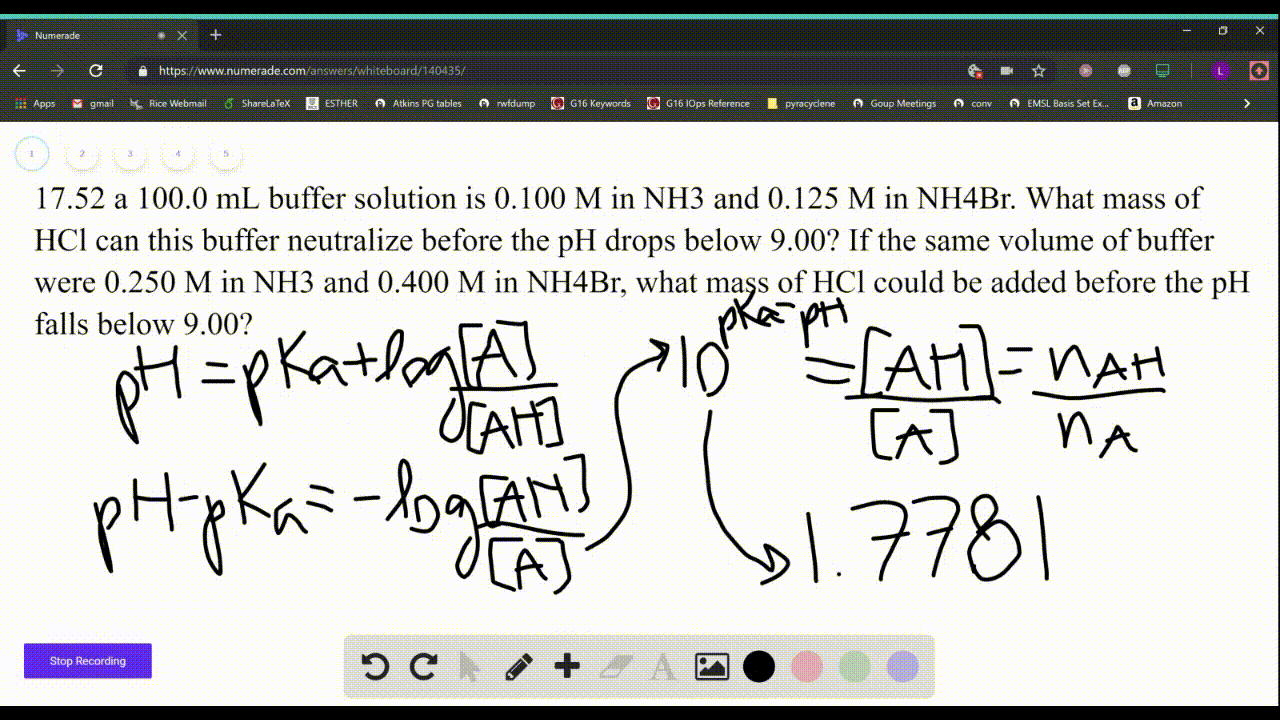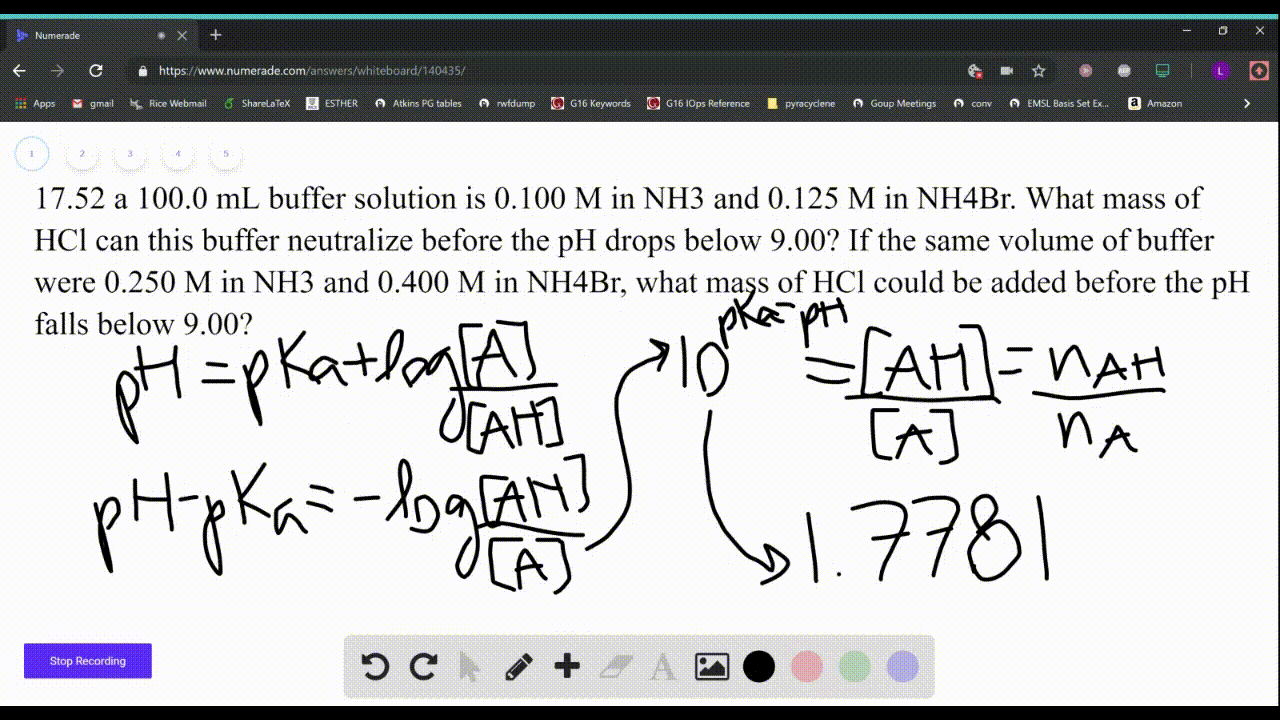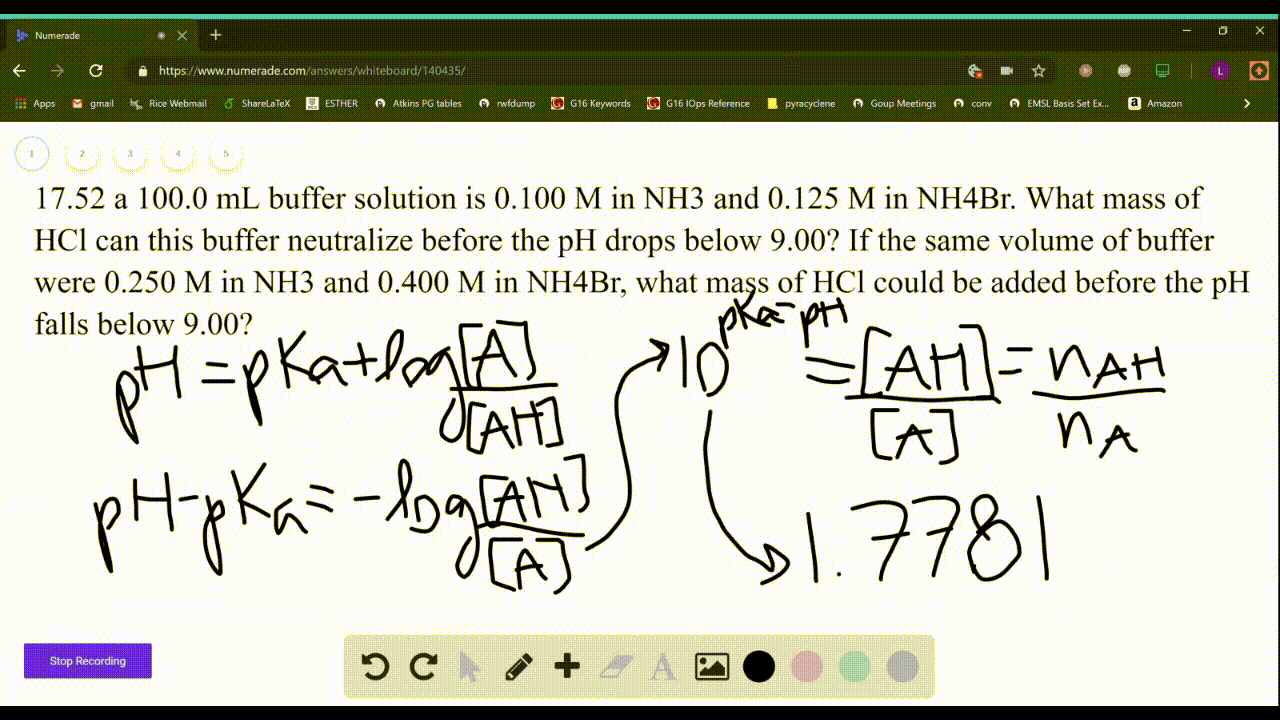
SOLVED: Which solution has the greatest buffering capacity?A) 0.335 M NH3 and 0.100 M NH4ClB) 0.085 M NH3 and 0.090 M NH4ClC) 0.540 M NH, and 0.550 M NH4ClD) 0.200 M NH,
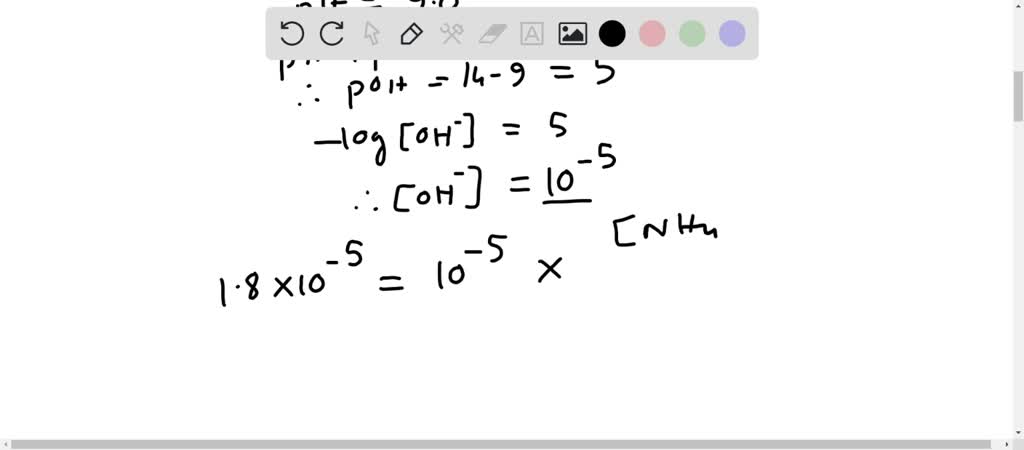
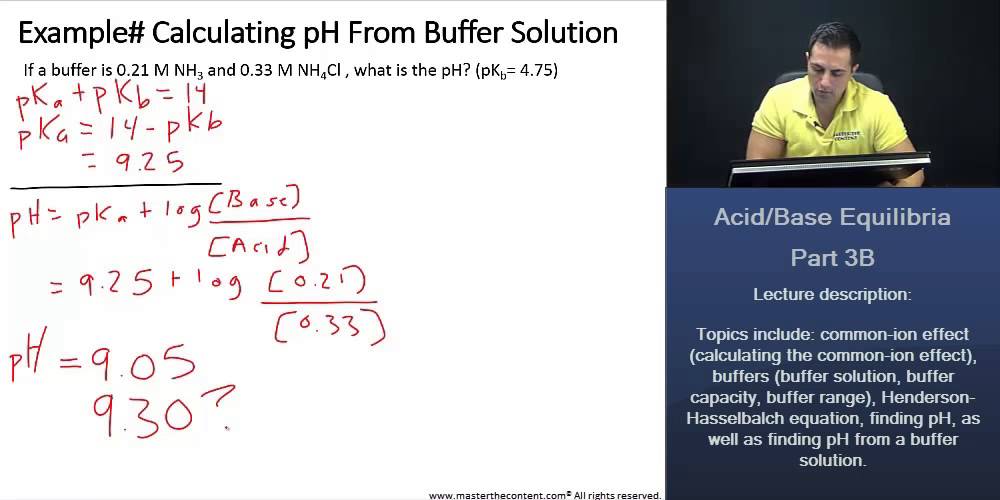
SOLVED: How many moles of NH4Cl must be added to 2.0 L of 0.10M NH3 to form a buffer whose pH is 9.00? (Assume that the addition of NH4Cl does not change